How a Product Design Company Can Humanize Medical Device Design: A Chat with THRIVE Director of Industrial Design John White
When you think of medical product design, you don’t typically think “approachable” or “humanized.” Scary, stainless, or something out of Star Trek, maybe. Things are changing, however, according to the team at THRIVE. As the relationship between user and machine becomes more symbiotic in medicine, our product design company and medical product designers are looking at ways to give medical devices a friendlier, more personal appeal and functionality.
“Approachable” and “humanized” are how John White (our Director of Industrial Design) describes the aspiration of today’s most innovative medical devices, however. “We’re starting to see products with more sensual, organic shapes and forms; User Interface’s (UIs) that are more simplified and emotive,” he explains. “It’s a paradigm shift in medical product design that began about a decade ago, as more generalized design firms started lending their expertise to medical device product development, bringing a consumer needs-based approach to the industry.”
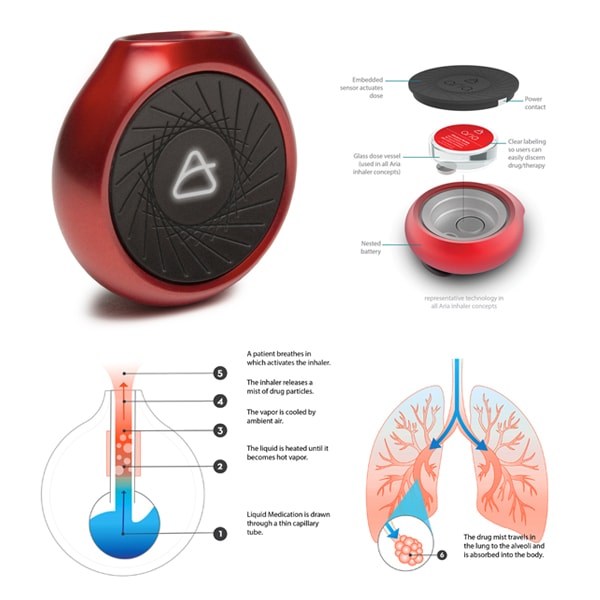
It’s also the philosophy White and his team brought to Aria, THRIVE’s re-imagination of the ubiquitous asthma rescue inhaler.
Plastic, bulky, and hardly discreet (including technology that’s at least 30 years old), the inhaler was due for an update. The key: new thinking tied to of-the-moment advancements. By utilizing vape technology, White’s team improved the device’s dosing delivery method and gave it a friendlier shape with best-in-class human factors and ergonomics. (The compressed gas mechanism of traditional inhalers meant the device had to be vertical. No more with vape tech.) THRIVE ultimately created four different versions of the inhaler for a concept project, each based on prominent personas and their needs: Youth, Contemporary, Sport, and Heritage. “Aria seeks to embody the delivery of the medicine in a way that intuitively connects with humans and becomes more ownable and less disposable,” White says.
White has long had a passion for innovation in the healthcare sector and enjoys solving the complex, real-world problems in the medical industry—ones that not only create a “new thing” but also translate to better patient experience and outcome. “There are constraints and criteria that go into medical product design that is above and beyond consumer product design,” he notes. “It’s a different animal in that you’re trying to solve hard-core usability needs.”
At THRIVE, we know that humanizing medical devices is difficult. “Stringent criteria that have to be met in medical product design to be both usable and innovative. It requires a lot more upfront contextual inquiry, analysis, planning, and strategy. So, you start there, rather than that coming at the end,” White explains. “You understand the gravity of what you have to prove (regarding testing, users, materials, etc.), so you align your activities and deliverables, so they plug right into FDA submission requirements.”
Whether designing a traditional consumer product or a medical device, our product development process starts at the same place: with medical ethnography and contextual inquiry. If we’re working on a new surgery tool, for example, we’ll observe 20 to 30 surgeons performing surgery, with interviews and photo documentation to identify pain points for widespread opportunities. “Then it becomes an iterative process,” White says. “We identify those opportunity spaces and then develop concepts that solve the problem and mitigate sacrifice, add something new, and then prototype and take those back out to the same users.” Rounds of testing and refinement follow, but the underlying idea of the user/machine relationship drives the process. It’s always about improving the user’s experience, so everyone’s outcome is as favorable as possible.
“There’s a lot of new technology — and new thinking — that can be applied to medical device design, and in a myriad of ways,” White says. This project is a prime example of that; Aria won a 2016 Red Dot Design Concept Award, one of the most prestigious design awards worldwide. “The overarching goal of this exercise was to tailor what is ubiquitous today for the needs of different users tomorrow,” White explains.
The bottom line: In healthcare, when we start with the person we’re designing for, we can create better products — and a happier, healthier world.
Read more about Aria, its design, and technology in our Case Studies section.
If your company is ready to grow and needs the strategy, design, and product engineering to meet your goals and objectives, contact our team of experts at THRIVE today.