Why Use-Related Risk Analysis (URRA) is a Critical Tool in Developing Safe, Effective, and Easy-to-Use Medical Devices.
Use-Related Risk Analysis (URRA) examines the variability of human behavior in particular scenarios, making it essential for identifying and mitigating hazards that can cause potential harm. Through thoroughly considering the medical device’s unique context, including the use environment, user characteristics, and specific operational scenarios, a URRA ensures a comprehensive risk assessment. This ongoing HFE process, bolstered by post-market evaluations, ensures that medical products meet regulatory safety standards, are user-friendly, and remain relevant and effective according to real-world user feedback and experiences.
This article is the second in a series of five articles focusing on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users.
____
Use-related risk analysis (URRA) is critical for safeguarding a product’s safety and enhancing its usability, effectiveness, and market success. By facilitating a deeper understanding of users’ interactions with a product, a URRA enables early identification of potential risks in the product development process, emphasizing implementing strategies to mitigate these risks proactively. As a Human Factors Engineer and Usability consultant, I’ll take you through the essentials of conducting a thorough URRA, underscoring its significance beyond mere human factors considerations. I’ll also highlight how THRIVE’s methodology for assessing use-related risks is customized for each product, depending on its specific use context, and focuses primarily on robust risk mitigation to ensure the highest safety and user satisfaction standards.
In my career, I’ve been fortunate enough to practice HFE in the aviation and medical device industries. I’ve focused on the design of products that are safe, effective, and easy to use and support end users in achieving their goals. My journey started with designing and evaluating cockpit displays and cognitively supportive technologies to strategize and conduct human factors activities on medical devices and pharma combination products. This experience has enabled me to comprehensively evaluate human interaction with medical and pharma products and effectively assess risk associated with product use. Throughout my time in human factors and usability engineering, I’ve conducted over 20 URRAs and have learned how to use them as a strategic tool to meet end-user needs and satisfy FDA regulators.
Understanding Use-Related Risk Analysis
The URRA is a structured and systematic process for evaluating potential hazardous situations and the harm they cause during critical aspects of product use. Compared to other analysis tools, such as design failure modes and effects analysis, the URRA considers the variation in human behavior, capabilities, and limitations. It’s a rubric — a detailed framework — that facilitates a deep dive into how a product is used in real-world scenarios, as evidenced by observed actual use and real-world data. It identifies where things might go wrong and proposes solutions to prevent those outcomes.
THRIVE’s URRA Framework: A Tool for Detailed Analysis
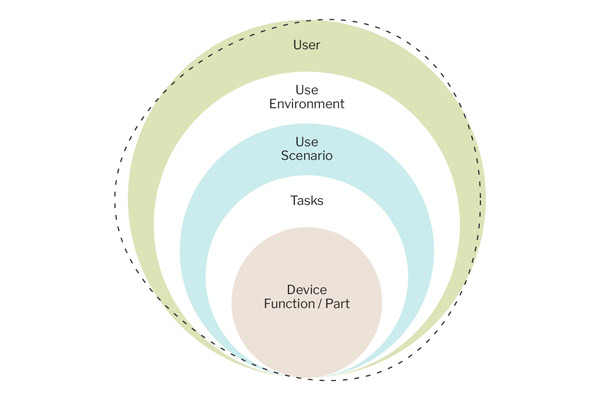
Our unique approach to URRA is pivotal in streamlining our risk analysis process for product design. This highly structured framework features situations, scenarios, and behaviors that capture various dimensions of user interaction. It encompasses:
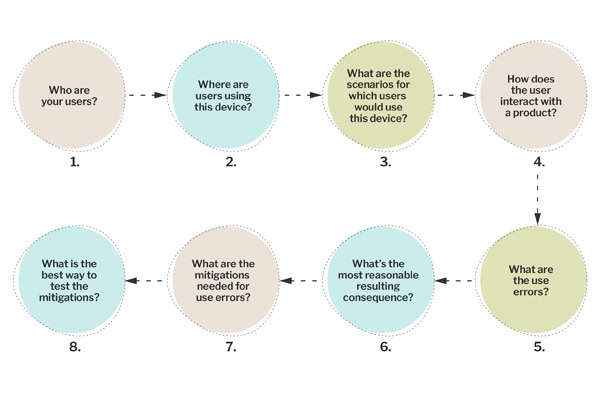
● An evaluation of your users. Thinking about who your users are and what they are using the product to accomplish, what their needs are, any factors around their emotions using the product, disease state, and the social aspects of using their product, and any limitations they may have that impact product use.
● An evaluation of where users are using the product. Thinking about the physical aspects of the environment (e.g., climates, pets, lighting, time of day the product is used) and the cognitive aspects of using the device (e.g., cultural, environmental, stigmas, employer policies impacting how a product is actually used).
● An evaluation of the use scenarios for which users would use the product. Consider whether there are different goals the user accomplishes with the product. What are all the things the user can do with the device within the context of its intended use?
● Evaluate how the user interacts with a product. What are the tasks the user must perform? What do those interactions look like? Are there gaps in time between interactions? Do the interactions match the user’s mental model? Are there transfer effects to consider?
● What are the use errors? Think about the ways in which users can make mistakes when performing each task. Can the user use the product incorrectly or for unintended purposes?
● What are the most reasonable resulting consequences? Working with clinical subject matter experts, think about the hazardous situations and harms resulting from the use error. Is it a severe consequence? Does this need (further) mitigation?
● What are the mitigations needed? Looking at the use errors and where and how they are committed, evaluate whether a mitigation exists, whether it is effective, and whether further mitigation is needed. Where does the mitigation need to go (i.e., where on the product, and can it be mitigated through a change to the design of the device?).
● What is the best way to test the mitigations? Think about how each mitigation can and should be tested. Which can be validated via simulated use testing versus other methods, such as knowledge tests?
This structured format allows teams to systematically analyze and assess critical facets of user interaction, ensuring no stone is left unturned in pursuing user safety, rewarding experiences, risk mitigation, and product usability.
Tailoring Your URRA to a Specific Product
A one-size-fits-all-products approach does not apply to URRA. The methodology is adaptable, requiring adjustments based on the nature of the product, its context of use, and the appropriate granularity of the analysis. Factors such as the environment in which the product will be used, the users and pertinent characteristics, the scenarios in which the product will be used, and their limitations play critical roles in effective analysis and assessing use-related risk at the appropriate level of granularity.
At various stages of product design and development, you may only be able to assess risk at the workflow level. This high-level thought exercise is still valuable as you may have enough information to anticipate specific use errors based on experiences assessing other medical devices and the current design. It also enables you to start identifying usability requirements that are helpful for the product design. Depending on the nature of the product, a much more detailed analysis may be warranted. Going through each button click and specific interaction may be needed to fully understand the usability challenges and potential use-related risks that must be mitigated.
At THRIVE, our bespoke approach ensures that the risk analysis is as relevant and actionable as possible.
Moving Beyond Summative Assessments
A Use-Related Risk Analysis (URRA) has traditionally been a foundation for informing summative assessments and pinpointing critical tasks required in an HF validation study. Yet, the breadth of its application surpasses this initial purpose. When implemented holistically, a URRA uncovers the effectiveness of various mitigation strategies. A URRA also helps prioritize the necessary actions and advocates for specific design modifications aimed squarely at curtailing use errors. This prioritization is intricately linked to the identified level of risk and severity accompanying different user tasks.
By integrating a URRA early and throughout the product design and development (D&D) phases, teams gain a nuanced understanding of potential user interactions and the risks these may entail. This insight allows for strategically allocating resources towards addressing areas of high risk or severe consequences first, ensuring that mitigation efforts are efficient and impactful. Furthermore, URRA fosters an environment of proactive risk management, where potential issues are addressed through effective user interface design before they manifest in real-world scenarios.
A URRA embeds safety and usability considerations into medical device design from the outset. By systematically assessing and re-evaluating risks at each stage of product development, URRA ensures that D&D endeavors comply with safety standards and FDA guidance and deliver a user-centered product.
The Strategic Simulation of Tasks
A vital aspect of a URRA is thinking through how various use steps or tasks can be simulated to evaluate potential risks appropriately. Understanding how best to simulate various aspects of the test environment and use scenarios, whether physically with equipment, lighting, sounds, and corporeal tissues or representing the cognitive stress and workload. Recognizing that resources — time and financial — are limited, URRA enables teams to strategize effectively, focusing simulations on areas that provide the most significant insights into use-related risks and the most effective way to validate risk mitigations. This pragmatic approach ensures that the analysis is both thorough and efficient.
Updating Your URRA Post-Summative and Post-Market Evaluation
The role of your URRA continues after the summative assessment. It’s a living document that should be updated to reflect insights gained from the final evaluations to evidence validation of risk mitigations and post-market assessment of risk. A quality system tracks complaints and other field data for big companies, providing information to inform URRA updates. By maintaining a current and accurate URRA, quality teams can better track specific usability issues, knowing precisely what to look for and how to identify problems more effectively in the post-market phase.
Why Use-Realted Risk Analysis (URRA) Matters
Use-Related Risk Analysis (URRA) focuses on the user’s interaction with the product. Unlike other risk analysis methodologies prioritizing technical or mechanical aspects, a URRA places the user at the forefront of the evaluation process. Doing so uncovers potential risks tied to how individuals use, misuse, or fail to use the product in real-world scenarios.
This user-centered approach allows URRA to identify the obvious residual risks and those that are less apparent or could emerge from unforeseen user behaviors. It considers the full range of human factors, including cognitive, physical, and even social and emotional aspects of a user’s interaction with a product. By examining how different types of users—spanning various ages, abilities, and backgrounds—engage with the product, URRA can pinpoint specific interactions that might lead to misuse or errors.
Furthermore, URRA’s emphasis on user interaction aids in developing more effective mitigation strategies. It provides a comprehensive understanding of why certain risks may occur, informing the creation of targeted interventions. These can range from design changes that make the product more intuitive to use to enhanced training materials that better prepare users for safe operation.
A URRA leverages a deep understanding of user-product interaction to illuminate risks that other tools might overlook, offering a robust framework for developing strategies that effectively mitigate those risks. This approach improves product safety and contributes to a more user-friendly design, ultimately leading to higher user satisfaction and product success.
Whether you’re at the helm of a startup or part of a large corporation, embracing URRA can be a game-changer, ensuring that your products meet the highest safety and usability standards.
____
OTHER ARTICLES IN THIS SERIES
If you missed any previous articles in this series on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users, you can find them here:
____
Ready to Scale and Conduct Use-Related Risk Effectively?
Please submit an inquiry using the below form. A member of our team will contact you shortly.
____
HUMAN FACTORS ENGINEERING AT THRIVE
THRIVE’s Human Factors professionals have decades of experience applying Human Factors to products ranging from medical devices used by specialized healthcare professionals in clinical environments to combination products used by laypeople in the home.
If it’s your first time applying Human Factors and you need a comprehensive end-to-end Human Factors program, we’ll scope out the program and conduct the activities on your behalf, leaving you time and resources to focus elsewhere. If you’re a resource-constrained HFE professional, we’ll provide the teamwork, collaboration, and support to help you meet your goals. Or, if you want a final sanity check to ensure you’ve met the latest and greatest expectations, we’ll do that, too.
ATLANTA | CHICAGO