Three Reasons Task Analysis is Critical for Delivering Excellent User Experiences in Medical Devices.
This article is the first in a series of five articles that focuses on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users.
As Human Factors and Usability Engineering consultants, we’ve assessed use-related risks for various medical devices and pharma products. There is incredible value in systematically assessing risk throughout the design and development process. This article covers the first step in determining use-related risk: conducting a thorough and effective task analysis.
Task analysis is a crucial aspect of the user-centered design process. It involves breaking down complex tasks into smaller, more manageable steps to understand the workflow and potential user pain points. This enables designers and engineers to know how their product or service will be used in the real world and make informed decisions about design requirements, principles, and directions. In this article, we explore how task analysis can illuminate unclear workflows, identify shortfalls in user testing, and allow for systematic risk mitigation.
As a biomedical engineer by training and a human factors engineer by trade, I have worked on every phase of the FDA waterfall product development process, including upstream R&D, downstream verification, validation planning, and testing. I’ve developed and leveraged various engineering, design, and regulatory control documentation as part of this work. I have found task analysis to be one of the most valuable documents for informing user-centered design.
Task analyses enable user-centered design that results in an enjoyable product rather than serving only as a requirement for safety and risk.
When utilized early, task analysis aims to open the door to ideation in targeted areas of need and decision-making around specific use cases. It also paints a detailed picture of seemingly mundane tasks that users may need to clarify to achieve their end goal.
Completing a task analysis may seem tedious—and for complex devices, it can be—but taking the time to do a complete analysis of how users would ultimately like to use a product or service pays back dividends for three reasons:
1) Task Analysis highlights unclear workflows in using a product or service.
2) Task Analysis allows you to pinpoint shortfalls in your product or service more precisely when conducting user testing and data collection.
3) Task Analysis allows you to mitigate risks in a systematic and adaptable way.
What is a Task Analysis, and Why is it Important?
Before discussing the three reasons why task analysis is critical in the user-centered design process, let’s define task analysis. Task analysis breaks down use scenarios into more granular tasks and subtasks that users perform to interact with a particular product or service.
The analysis structure promotes consideration of the environments where the tasks will be performed, as environmental factors may influence the user’s ability to perform those tasks. It also encourages consideration of the end users, as that will impact how they perform those tasks and serve as inputs to a user-friendly design.
Dissecting the use scenarios in this way will help you better understand where use errors can occur and where to modify the design to mitigate those errors. It will also serve as a rubric for your usability evaluations.
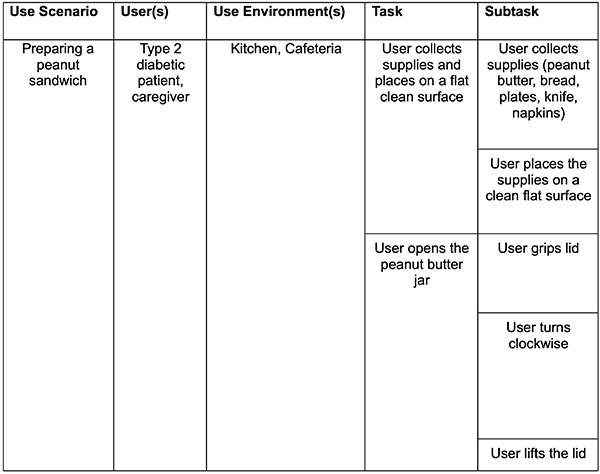
Task Analysis Example (Making a Peanut Butter Sandwich):
1. Task Analysis Highlights Unclear workflows when they are created.
As developers and manufacturers, we may take for granted how intuitive our design is because we know it intricately—we designed it, after all!
Building a task analysis makes unclear workflows pop out at you.
When executed early in the design process, task analysis enables your design and engineering team members to consider all aspects of the UX design to create a safer, more usable, and more enjoyable overall product experience.
Task analyses also highlight workflows that may harm the user or the patient, laying the groundwork for design decisions that effectively mitigate use errors and use scenarios to include in your usability evaluations.
Building a task analysis may seem tedious. Should we really outline every detailed action a user may take while interacting with my device or interface? In my experience, the answer has always been a resounding “yes!” The “why” will become very apparent as you work through each specific task in smaller steps.
You will begin to identify possible areas of confusion for the user. As you dissect workflows into tasks, workflow shortfalls will become evident in how you describe a particular task or subtask. If your wording becomes confusing, clunky, or verbose, it may indicate an area for improvement or modification of your product or interface.
Taking a fine-tooth comb to your device and rigorously analyzing individual steps to establish a baseline will allow you to refine your design and catch simple usability issues and potential risks early in the design process (when it is much cheaper to change, test, and fix).
The task analysis is the backbone for device design, use-related risk reduction, validation requirements, documentation, and testing for medical device products. You cannot assess usability comprehensively without one. It will enable your team to capture critical tasks, possible misuse scenarios, and potential hazards from a user-centered perspective. Task analysis allows your engineering and design teams to focus their energy on mitigations for the most potentially harmful situations.
2. Task Analysis allows you to pinpoint usability shortfalls during user testing.
What is a test or project without a grading rubric? With a complete task analysis, you have identified the user’s actions to use the device safely and effectively. It is essentially “showing your work,” allowing you to determine the exact moment of unclarity that causes a user to slow down, become stuck, or make a mistake in a workflow.
Without a solid task analysis, you will likely experience many headaches when conducting formative and summative testing, as knowing what to test and how to test it will be incredibly murky.
Having a well-done task analysis will illuminate shortfalls in design, labeling, IFU communication, and much more. Allowing you to peel back the layers as discrete steps in the user journey and identify the root cause enables focused problem-solving and decision-making for all stakeholders in the R&D process.
When tackling more complex workflows, task analysis can be supplemented with a “Perception, Cognition, Action” (PCA) analysis, allowing developers or manufacturers to systematically deconstruct the user’s interactions with the device.
3. Task Analysis allows you to systematically and adaptively mitigate risks.
Once possible misuse is captured, design and engineering teams can focus on mitigating these risks.
A team may prioritize the most disruptive or frustrating misuse cases for consumer-facing products first. This helps to reduce customer service center calls or unfavorable social media posts about design shortfalls in your product.
Your task analysis provides the foundation for identifying use errors and the resulting hazardous situations in medical products. Once these situations are outlined, potential harm to patients or users and the severity of that harm can be effectively assessed. With a clear picture of the possible harms, mitigations can be designed and implemented.
For high-severity harms, it is a requirement to implement and test risk mitigations in your product to prove it is safe and usable, referencing your task analysis as a “rubric.”
Impact on the user and consideration for misuse shouldn’t be made only when a product or service is released for the first time. It should also be used to evaluate updates to the design and track the impact of any changes to usability and use-related risk as devices are modified.
Keeping an up-to-date task analysis allows teams to consider and formally document use-related impacts without relying on the design team’s memory or working through hypothetical and unrealistic scenarios. Task analysis can also be used to effectively track source complaints from the actual operating environment and compare the performance of one device iteration to another.
When should you do a Task Analysis?
Task analysis should be done early in the design process and maintained as a living document as the device evolves. It should be an ongoing collaborative effort between the different functions in your organization that sometimes need to be included in the process but often slip through the cracks.
It can be helpful to involve experts in creating the task analysis. It is even better if they know how to champion the information to the proper teams and facilitate R&D decision-making based on the results.
Don’t underestimate the power of task analysis—whether you’re creating a biomedical device or a peanut butter sandwich, it can make a world of difference.
Contact us for help or guidance on creating robust task analyses, enabling human-centered design choices, or implementing human factors for your device, product, or service. We’re here to help!
____
OTHER ARTICLES IN THIS SERIES
If you missed any previous articles in this series on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users, you can find them here:
4. The Importance of Continuous Risk Assessment in Medical Device Design & Development
____
Ready to Scale and Conduct
Use-Related Risk Effectively?
Please submit an inquiry using the below form. A member of our team will contact you shortly.
"*" indicates required fields
____
HUMAN FACTORS ENGINEERING AT THRIVE
THRIVE’s Human Factors professionals have decades of experience applying Human Factors to products ranging from medical devices used by specialized healthcare professionals in clinical environments to combination products used by laypeople in the home.
If it’s your first time applying Human Factors and you need a comprehensive end-to-end Human Factors program, we’ll scope out the program and conduct the activities on your behalf, leaving you time and resources to focus elsewhere. If you’re a resource-constrained HFE professional, we’ll provide the teamwork, collaboration, and support to help you meet your goals. Or, if you want a final sanity check to ensure you’ve met the latest and greatest expectations, we’ll do that, too.
ATLANTA | CHICAGO