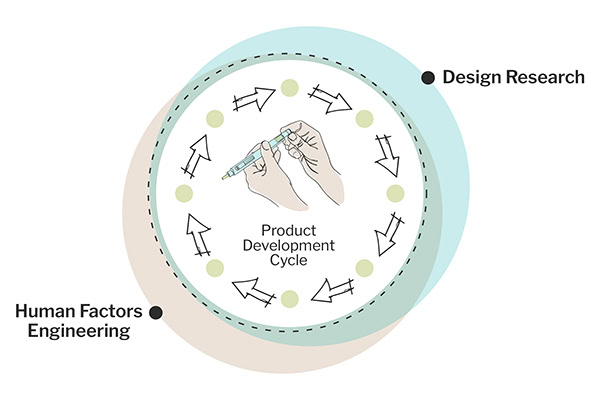
In critical medical device design and development, conventional risk assessment methods frequently leave risk evaluation until the last minute. Continuous risk assessment at every stage of new product development turns risk assessment into a potent instrument for well-informed decision-making and more robust product safety. In this article, we will explore the transformative benefits of ongoing risk awareness, guaranteeing that each advancement is well-considered, perfectly aligned with business goals, and adeptly navigates the intricacies of innovation. We also cover how THRIVE synchronizes risk management across various domains, broadening risk mitigation beyond safety to encompass business and usability risks.
Continuous risk management is not only advisable but imperative in the high-stakes realm of medical device design. Traditional models often defer risk assessment to the eleventh hour, but integrating it throughout the project lifecycle makes it a powerful tool for sharper decision-making and ironclad product safety.
With a career spanning the aviation and medical device industries, I’ve honed my expertise in Human Factors Engineering (HFE). My focus has been on designing products that are not only safe and effective but also user-friendly, supporting end users in achieving their goals.
In this article (the fourth in of a series of five focusing on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users), I’ll explain the advantages of continuous risk assessment, ensuring that every step forward is well-informed and fully calculated.
Continuous Risk Assessment: A Strategic Approach
Optimizing Time and Resources
Continuous risk management brings you a strategic edge with its capacity to optimize time and resources, using risk to prioritize project focus areas and effort (because you’ve identified areas of greater risk or areas that will need more exploration). By weaving continuous risk assessment throughout the project lifecycle, teams gain the foresight to spotlight critical areas needing urgent focus (or, for example, determining that by this time next year, you’re going to need to know whether to choose a particular design path or abandon it and go down a more traditional one) and judiciously channel funds where they’re most needed. This proactive stance mitigates the risk of eleventh-hour panic and subsequent shocks and guarantees meticulous oversight of every project facet — from timelines and budgeting to design nuances and functional usability.
Harmonizing Risk Management Across Domains
Several different risk assessments exist (e.g., clinical risk, design risk, manufacturing risk, use-related risk), and the design and development teams need to talk about these different forms of risks to determine how best to mitigate them (if we mitigate a design risk that may increase a use-related risk, or if we mitigate a use-related risk, it may increase a clinical risk). Embedding continuous risk assessment through the design, development, manufacturing processes, clinical trials, and safety assessments is challenging. Ongoing risk management is a unifying force, threading these different workstreams into a cohesive narrative that aligns seamlessly with overarching project ambitions and stringent regulatory frameworks. This synthesis is vital for fostering a holistic, integrated risk strategy that maintains consistency and coherence throughout all project phases. It also helps to inform better mitigation strategies for the product.
Expanding Horizons: Beyond Safety to Business and Usability Risks
While ensuring safety is undoubtedly crucial, particularly in medical device development, the scope of ongoing risk assessment extends far into business viability and user engagement. A product might be technically flawless, but its commercial prospects dim if it falls short of market demands or user expectations. Employing rapid, cost-effective testing with a handful of users (see Figure 1 below) can quickly unearth up to 95% of potential user errors, providing critical insights that allow teams to refine and adjust long before these pitfalls jeopardize the product’s success in the competitive marketplace.
Many people who work in medical device development see HF testing as expensive and time-consuming and want to avoid it when there is no use-related safety risk. Jakob Nielsen’s discount usability is a way to test these no-risk products and reduce the business risk associated with annoying usability issues that prevent users from purchasing or using the product.
Jakob Nielsen’s assertion that “Discount usability often gives better results than deluxe usability because its methods drive an emphasis on early and rapid iteration with frequent usability input” underscores the transformative power of simplicity and efficiency. The Nielsen Norman Group’s pioneering work on Discount Usability over the past two decades has redefined how many human factors and usability engineers approach user testing and design refinement.
By advocating for streamlined methods such as simple user testing with just 5 participants (although we use a minimum of 15 participants to increase the probability of detecting usability problems), paper prototyping, and heuristic evaluation, they’ve unlocked a pathway to cost-effective yet highly impactful usability insights.
To be clear, this is not how we conduct HFE in general — the main takeaway here is that there is a time and place for discount usability, particularly if you’re developing something that is not a medical device (e.g., software that doesn’t have use-related risk but is used by patients or HCPs, however, even then, you still need to do testing on it to make sure it’s usable even if it doesn’t have use-related risk). Doing cheap and quick usability tests can help us identify many issues that we want to do away with before going to market.
This approach can accelerate the identification of usability issues and lay the foundation for a culture of continuous improvement through iterative design cycles. However, it’s crucial to recognize the context of these methods; they excel in early formative testing, focusing on refining concepts and user interactions rather than comprehensive risk assessment. Yet, their effectiveness in swiftly uncovering usability pitfalls remains unparalleled, making Discount Usability a cornerstone of modern HFE practice.
Proactive Regulatory Engagement
Engaging early and often with regulatory bodies such as the FDA is critical, particularly when introducing cutting-edge therapies or technologies. Initiating dialogue at the outset of development demystifies regulatory expectations and ensures that your project is built on a foundation of compliance. These early interactions can be pivotal, offering a chance to adjust or radically shift strategies if the current approach may not secure regulatory approval, saving invaluable time and resources.
Strategic Business Risks and Decisions
Effective continuous risk assessment transcends safety and compliance, guiding strategic business decisions that define a project’s trajectory. Choosing between pioneering new technologies and opting for established methods involves a nuanced balance of risk and reward. Early engagement with regulatory processes is crucial; if innovative solutions stall in regulatory limbo, switching to reliable, proven technologies might be prudent, safeguarding the project’s viability without stifling innovation.
Design Innovation and User Safety
Consider the impact of seemingly minor design changes through the lens of ongoing continuous risk assessment in the medical device industry. For instance, altering design aspects to improve aesthetics may impact how the user uses the product and can lead to more use-related safety consequences. Continuous risk evaluation empowers design teams to foresee such pitfalls and deliberate whether the benefits of aesthetic changes outweigh potential safety risks, ensuring that every design decision enhances user safety without unintended consequences.
Embracing the Power of Continuous Risk Management
The importance of ongoing risk management cannot be overstated—it’s a linchpin in the world of product development, crucial for ensuring safety, compliance, and, ultimately, the market success of a product. This proactive strategy allows businesses to adeptly maneuver through the intricacies and uncertainties of bringing a new product to market with remarkable precision and assurance. By embedding continuous risk assessment into every stage of the product lifecycle, from the drawing board to the final market introduction, companies ensure that their innovations are secure, compliant, and ideally attuned to users’ expectations and the business’s strategic aims.
Adopting this approach transforms risk management from a defensive tactic into a strategic asset, enabling teams to remain nimble, forward-thinking, and innovative. It turns potential vulnerabilities into springboards for refinement and success, illustrating that the true strength of a business lies not just in creating products but in smartly navigating the risks associated with their development and deployment.
____
OTHER ARTICLES IN THIS SERIES
If you missed any previous articles in this series on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users, you can find them here:
____
Ready to transform your project’s risk management strategy into a proactive force for success?
Discover how THRIVE’s human factors engineering team can elevate your project to new heights. With decades of experience across a spectrum of products — from specialized medical devices to consumer goods — we tailor our approach to meet your specific needs.
Whether it’s your first foray into human factors or you’re seeking a final sanity check, we’ve got you covered. Let’s turn challenges into opportunities and ensure your venture thrives in today’s dynamic landscape. Reach out now, and let’s embark on this journey together!
"*" indicates required fields
____
HUMAN FACTORS ENGINEERING AT THRIVE
THRIVE’s Human Factors professionals have decades of experience applying Human Factors to products ranging from medical devices used by specialized healthcare professionals in clinical environments to combination products used by laypeople in the home.
If it’s your first time applying Human Factors and you need a comprehensive end-to-end Human Factors program, we’ll scope out the program and conduct the activities on your behalf, leaving you time and resources to focus elsewhere. If you’re a resource-constrained HFE professional, we’ll provide the teamwork, collaboration, and support to help you meet your goals. Or, if you want a final sanity check to ensure you’ve met the latest and greatest expectations, we’ll do that, too.
ATLANTA | CHICAGO