Six Steps to Determine if Your Risk Mitigation Strategies Are Effective in Your Device Development Programs
Integrating effective risk mitigation strategies into a medical device’s design and development is vital for designing devices that operate effectively in high-stress environments like hospitals. These risk mitigation strategies reduce user error risks and improve safety by making devices more intuitive and user-friendly and accommodating nurses’ time pressures. A comprehensive understanding of user behavior and context is crucial for designers to develop practical, reliable interventions for real-world medical applications, effectively mitigating human factors risks.
This article is the final part of an article series that assesses use-related risk and its value to your medical device, service, company, customers, and end users. In this article, we delve into the crucial aspect of ensuring the effectiveness of risk mitigation strategies related to a device’s design or interface, especially in high-stress environments like hospitals. Effective mitigations reduce the risk of user error, which can have severe consequences, particularly in critical care settings. The key to implementing risk mitigation strategies is understanding human behavior in context, such as recognizing that nurses often operate under time constraints and may not thoroughly read screen instructions. This understanding helps design effective, user-friendly interventions (mitigations) that improve human performance and decision-making.
As a Human Factors Engineer (HFE) and Usability consultant, I’ll provide you with actionable steps and advice for understanding user context, analyzing errors and implementing appropriate and effective mitigations, testing mitigations proactively, optimizing usability through user feedback and iterative testing, effectively simulating the use scenario and streamlining medical device development.
In this article, I’ll highlight how THRIVE’s risk mitigation methodology is customized for each product, depending on its specific use context and work environment, to ensure the highest safety and user satisfaction standards.
1. Understanding User Context and Error Analysis
Ensuring the safety of medical devices involves a comprehensive understanding of the user’s characteristics, the operating environment, and the context of use.
Delivering usable medical devices to market with no safety risks requires thoroughly considering a user’s physical and cognitive conditions and the specific environmental and other contextual factors impacting device interaction needed.
End users in a particular use context (such as a patient undergoing nightly dialysis) might be abruptly woken by their alarming device and need devices that accommodate these aspects of use — like grogginess and uncorrected vision when awoken from sleep. Additionally, adaptations for sensory impairments, like larger fonts or audio feedback, are essential to cater to common issues such as reduced vision or hearing, particularly in elderly patients. In high-pressure settings like ICUs or operating rooms, where healthcare professionals face extreme stress and frequent interruptions, devices should incorporate auditory cues to facilitate quick and accurate understanding and response by the users. Integrating the context of how a device will be used into the design of the device will support the user’s workflow and reduce use errors.
One way to do this is to conduct user research to understand your users, the setting in which they’ll use the device, and how they work in that setting. Conducting concept evaluations and early usability testing can help identify various human factors considerations that need to be accounted for in the device’s design.
2. Analyzing Errors and Implementing Risk Mitigation Strategies
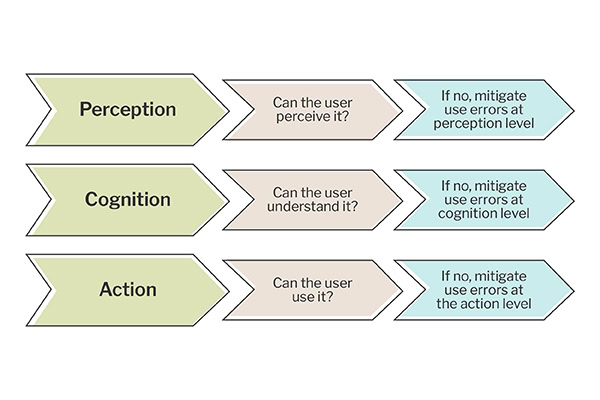
You need to understand what is causing the error (by knowing the root cause of the problem through risk analysis, you can correct or mitigate it at the root rather than somewhere else) and what kind of error it is (leveraging the PCA model). Leveraging the PCA model, you can understand whether it’s an error in perception, cognition, or action–you want to avoid mitigating at the action level if the issue is with perception. If the user can’t even see the button because it’s located on the screen in a place where they don’t expect to find it or because it doesn’t have enough contrast with other parts of the screen, then you know you need to make a design change to make the button visible to the user. If the user sees the button but doesn’t understand the outcome of clicking it or that they need to click it to advance to the next step or screen, then you know it’s not an issue with perceiving the button. Therefore, you would mitigate this by changing the button label or otherwise making it obvious what clicking the button will do. If the user can see the button and know when to press it, they keep pushing the wrong button because it’s too close to the button they intended to press or because the buttons are too small. You can mitigate this by changing the button size and distance between the buttons.
3. Designing for Error Prevention
Once you understand the setting in which the device will be used and the nuanced characteristics of the users, you can understand when and where they are likely to make a human error and which risk mitigation strategies may not be effective for this user group.
For example, adding language to the IFU may not be an effective risk mitigation strategy if we know that the IFUs are typically not read by the users because (a) they are kept in a cabinet that isn’t easily accessible or (b) they don’t have time to read them in an emergency. Understanding these potential risk factors will help you understand the use errors that could be made and mitigate them more effectively.
Preventing human error involves employing design and the application of human factors to make the correct actions obvious to reduce the chance of error. For instance, uniquely shaped connectors for medical tubing ensure only proper connections are made, preventing dangerous misconnections and effortlessly guiding users to the correct actions. Some medical tubing connectors are keyed so that only one tube can go into a slot, allowing only the correct tube to be connected.
Redundancies in medical devices, such as double-checking mechanisms or requirements for secondary confirmations on critical actions, act as safety nets to human failures, catching and correcting errors before they lead to severe consequences. These features are particularly vital in high-stress environments where the probability of human error is heightened.
4. Proactively Testing Your Risk Mitigation Strategies
One way to ensure that your risk mitigation strategies are effective is by testing them. It’s better to know earlier than when it’s too late whether risk mitigation will work. Sometimes, some mitigations will work, but we are not the users, and they may not work in their home or work environment. Furthermore, we may have an effective risk mitigation strategy, but we’ve mitigated it at the wrong level. We may have mitigated at the cognition level when the mitigation should be at the action level.
An example may be that the user doesn’t press the button they should. It may be because the user doesn’t know that they need to press it when they know this, but they cannot because it requires too much force to push. This is also where conversing with the users helps you understand their thought processes and why they committed the use error. In this case, you’ll get insight into whether it’s an issue with knowing that they need to press the button versus knowing but not physically being able to push it. Early testing of our mitigations will help us understand whether they are effective and, if not, how to make them as such. Also, leverage existing data. Look at similar devices and their use error mitigations. Are they effective? Leverage that information in your design and risk mitigation strategies to ensure effective mitigation.
5. Conducting Simulations and Scenario-Based Testing
Simulating real-world conditions is indispensable for evaluating medical devices’ usability and use-related safety in their operating environments. This means physically recreating the operating environment in the testing facility to simulate the physical aspects of the use environment, such as lighting, noise, even temperature, and spatial constraints, as there might be in a dialysis clinic, for example. This also means recreating the use environment from a cognitive standpoint. Think about an emergency use case in which a nurse is part of a medical team working on a patient as the patient is crashing. You have alarms, hysterical family members, and doctors yelling at you; you have to go and get life-saving medication from the Pyxis as quickly as possible. All those factors may impact your ability to identify the correct medication for this crashing patient compared to a calmer environment in which you don’t have the time constraints, the emotions of trying to save the patient, etc. To understand the real-time use errors a nurse can make under those stressors, you have to simulate the setting of a crashing patient. Furthermore, to effectively test the risk mitigations designed into a Pyxis station to prevent incorrect medication from being dispensed, you have to simulate the setting accordingly to assess its safety management systems and control measures.
Real-world data is invaluable for validating the effectiveness of your risk mitigation strategies. Performance reviews, ergonomic audits of existing devices, and analysis of customer feedback and adverse event reports provide insights into mitigation success and areas needing improvement. For instance, higher recognition rates for auditory alarms over visual ones indicate more effective design strategies. This data is essential for fine-tuning device safety and functionality to ensure they align with user needs and conditions.
Sometimes, It may not add value to simulate all aspects with 100% fidelity or test an entire workflow. There are times when it’s necessary to test the risk mitigation effectively. Think about when the accuracy of setting up a device impacts the efficacy of the therapy delivered to the patient. If we only test the therapy delivery portion, you’re not allowing use errors and their mitigations associated with setup to be tested. I was conducting an evaluation in which users consistently committed puzzling use errors given the clinical context. Specifically, they were disconnecting connections that would expose them to corporeal fluids. During root cause analysis, I inquired about this, and the participants told me that they would never do this in a natural setting because of the exposure to fluids. When I probed when they made the use error during the evaluation, it became clear that the fluids weren’t simulated with a high enough fidelity, and they treated the fluid as water rather than corporeal fluid. Participants must be placed into a simulated use environment that delivers the necessary level of fidelity to trigger the same behaviors as they would in the clinical operating environment.
6. Optimizing Devices Through User Feedback and Iterative Testing
Following scenario-based testing, detailed feedback from end-users like nurses, doctors, and medical technicians is essential. This feedback helps assess how effectively the device’s safety features support users in performing their tasks effectively and safely. The information collected is pivotal for making iterative adjustments to the device, including modifying limits, altering feedback mechanisms, or redesigning user interface elements to enhance clarity and usability.
This is because (a) we need to understand what users think about when they use the device and to understand their thought process as they commit use errors and interact with the device, and (b) some risk mitigations are complex to test, such as confirmation screens–you can corroborate observation and even eye tracking data with user anecdotes about the information they understood from the confirmation screen.
Iterative testing and user feedback are fundamental to the human factors engineering process of medical device design, aimed at maximizing usability and ensuring adherence to safety standards. The HFE process starts early in the design phase (at least, it should: read how bringing HFE forward in D&D can help circumvent costly design blunders) and extends throughout the product development cycle, allowing for early identification and mitigation of usability issues. Each testing phase is followed by collecting extensive feedback from various user groups, which is crucial for understanding the diverse challenges and needs across different medical settings.
Update your Usability Risk Assessment (URRA) with data from the latest tests. This document is crucial for guiding subsequent design modifications, detailing how each round of feedback and testing results in substantial improvements or the introduction of new safety features. URRAs also serve as a vital record for justifying design changes, particularly those addressing identified usability risks, which is essential for regulatory compliance and stakeholder communication.
Determining the effectiveness of risk mitigation strategies in medical devices is a multi-step process involving an in-depth understanding of user behaviors, testing, and continuous refinement based on user feedback and real-world data. By focusing on applying human factors and the real-world contexts in which devices operate, designers can create products that meet clinical needs and enhance patient safety and usability. This proactive approach improves product quality, ensures compliance with rigorous regulatory standards, and optimizes the performance of the human-and-device system.
____
OTHER ARTICLES IN THIS SERIES
If you missed any previous articles in this series on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users, you can find them here:
4. The Importance of Continuous Risk Assessment in Medical Device Design & Development
____
Ready to transform your project’s risk management strategy into a proactive force for success?
Discover how THRIVE’s human factors engineering team can elevate your project to new heights. With decades of experience across a spectrum of products — from specialized medical devices to consumer goods — we tailor our approach to meet your specific needs.
Whether it’s your first foray into human factors or you’re seeking a final sanity check, we’ve got you covered. Let’s turn challenges into opportunities and ensure your venture thrives in today’s dynamic landscape. Reach out now, and let’s embark on this journey together!
"*" indicates required fields
____
HUMAN FACTORS ENGINEERING AT THRIVE
THRIVE’s Human Factors professionals have decades of experience applying Human Factors to products ranging from medical devices used by specialized healthcare professionals in clinical environments to combination products used by laypeople in the home.
If it’s your first time applying Human Factors and you need a comprehensive end-to-end Human Factors program, we’ll scope out the program and conduct the activities on your behalf, leaving you time and resources to focus elsewhere. If you’re a resource-constrained HFE professional, we’ll provide the teamwork, collaboration, and support to help you meet your goals. Or, if you want a final sanity check to ensure you’ve met the latest and greatest expectations, we’ll do that, too.
ATLANTA | CHICAGO