Tailoring Use-Related Risk Analysis (URRA) for Enhanced Risk Mitigation and Usability in Medical Device Design and Development
Medical device design and development is a complex and challenging process that involves various stages, from idea generation to final production. One critical aspect of new product development is risk mitigation. Use-related risk analysis (URRA) is essential in achieving this, as it helps identify potential hazards and assesses their impact on product safety and performance.
A comprehensive approach to risk analysis is not just a regulatory requirement — it’s a strategic necessity. Use-Related Risk Analysis (URRA) is a dynamic and in-depth methodology that addresses various potential risks associated with product use. At THRIVE, we have developed a unique approach to URRA to meet the needs of our client partners and ensure product safety and usability while delivering on business goals. This blog discusses the importance of use-related risk analysis in medical device design and development and how it can help mitigate risks and enhance usability.
Tailoring URRA to Product Needs
Every product has challenges and nuances, and adopting a tailored approach to Use-Related Risk Analysis (URRA) in medical device design and development is essential. At the outset of our URRA process, we engage in a deep dive to fully understand the product’s intended use, the environments in which it will operate, and the various types of users who will interact with it. This initial exploration is crucial as it informs the level of detail and the specific areas we need to focus on during the analysis.
The granularity of our analysis can vary significantly depending on the product’s complexity and stage in the development lifecycle. A detailed, granular approach is essential for products early in their design phase or those with intricate mechanisms and multiple user interfaces. Here, we meticulously deconstruct every conceivable user interaction down to micro-steps. This thorough examination helps uncover subtle risks that might not be evident in a high-level analysis and ensures that every potential user error is considered for risk mitigation and addressed.
Conversely, a broader analytical approach might be more appropriate for products nearing the final stages of their design or those with simpler interfaces and fewer user interaction points. In these cases, we ensure that all major risk factors have been identified and adequately mitigated. Risk mitigation might involve reviewing and updating previous analyses to reflect any changes made during the design process, ensuring that the product is safe for use and that all significant risks are well-documented before market release.
The Role of Organizational Intent in Shaping URRA
Our client partners’ needs and their products play a critical role in shaping the scope and focus of our analysis for the URRA. Understanding what the organization plans to do with the analysis helps us tailor our approach accordingly and ensure that the URRA is fit for purpose.
Let’s consider that the URRA will be used early in the design and development process to identify usability improvements and areas where use-related risk must be further mitigated. In that case, we will use a more granular approach, analyzing the lower-level use steps, identifying foreseeable use errors, evaluating the effectiveness of the risk mitigations (and thus prioritizing which ones need further improvements), or identifying gaps where risk mitigation is needed.
We use the URRA to drive subsequent design and development activities versus the case where we are being asked to either review or develop the URRA for a product whose design is nearly frozen. In this case, we may shift our approach to look at higher-level tasks rather than lower-level steps and ensure that there are mitigations in place (before validation) for the critical use errors or to validate those mitigations via testing. We are doing a final risk assessment to prove that the device has been designed safely and effectively, not to identify further usability improvements.
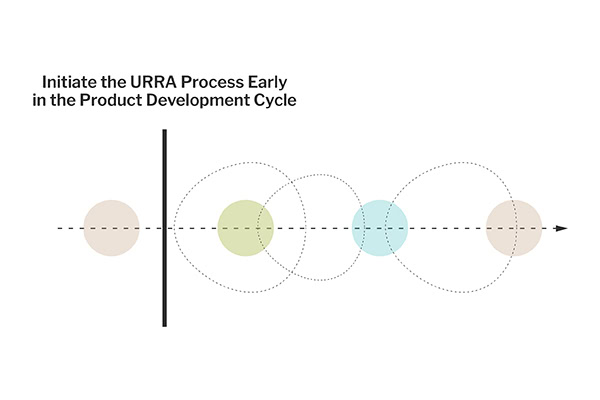
In both cases, initiating the URRA process early in the product development cycle is critical. Early engagement allows for a more informed design process and helps set a clear and effective course for subsequent risk analysis activities. It ensures that risk mitigation efforts are integrated throughout the product development rather than being an afterthought, leading to safer and more user-friendly products.
Early Stage Risk Mitigation
From the inception of the product’s development, we worry about use-related risks and how to mitigate them. This phase is critical as it sets the foundation for the safety and usability of the product throughout its lifecycle. To achieve this, we conduct a comprehensive analysis that thoroughly examines every conceivable user error. This analysis might involve exploring errors in user perception and cognition resulting from a user not understanding the necessary action or errors of action resulting from physical design flaws like a button being too small to operate effectively.
Our approach at this early stage involves simulating various usage scenarios to unearth any design flaws that could lead to user errors. By identifying these risks early, we can collaborate closely with design teams to remove these flaws through design changes, thus enhancing the overall safety and usability of the product. This proactive risk mitigation strategy is crucial for meeting safety standards and reducing the cost and time associated with redesigns later in the medical device design and development process.
Design and Usability Improvements
While not all usability issues pose direct safety risks, they are vital for ensuring user satisfaction and the product’s overall success. During the URRA process, we emphasize the product’s real-world application. By understanding how the product is used, we can identify usability challenges and opportunities for enhancement.
These improvements aim to make the product easier to use, more appealing, and more efficient, meeting or exceeding the high expectations of end-users and stakeholders, for example, refining the interface to reduce the number of steps in a common task or enhancing the ergonomic design to prevent user fatigue during prolonged use. These usability enhancements often translate directly into improved user satisfaction and increased product adoption.
Late Stage: Final Risk Analysis and Validation
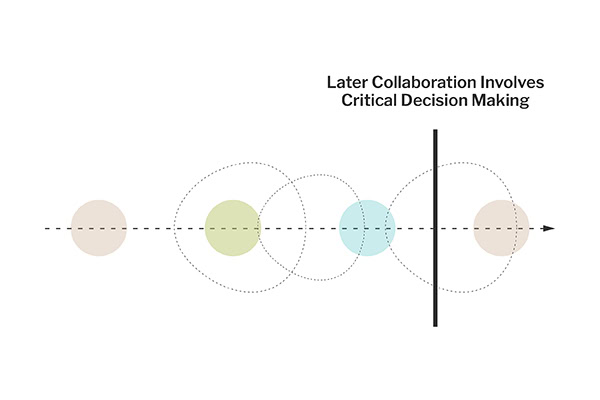
When our involvement begins closer to the conclusion of the product development process, our role in conducting the URRA remains crucial. At this late stage, the focus shifts to a high-level review of the risks identified and mitigated earlier in the design and development process. We collaborate with the organization’s clinical team to examine the practical implications of residual risks and discuss how to manage these in a clinical setting.
This stage often involves critical decision-making where the benefits of specific design elements are weighed against their potential risks. Decisions made during this phase are informed by a deep understanding of the clinical environment where the product will be used, requiring a balance between clinical judgment and the practical guidance provided in the product’s documentation.
The final risk analysis ensures that all possible risks have been considered and addressed. It validates that the mitigations are effective and that the product meets all required regulatory standards before it goes to market. At this stage, validation testing with users or other forms of empirical evaluation ensures that the product is safe and effective for its intended use.
Collaboration with Clinical Teams
The Use-Related Risk Analysis (URRA) process is a deeply collaborative effort that requires ongoing interaction with clinical teams. This collaboration is vital as it bridges the gap between risk assessments and practical, real-world applications in medical settings. By working closely with clinicians with first-hand experience with the product’s conditions, we can ensure that the URRA reflects actual use scenarios, not just controlled environment tests.
This partnership allows us to gain insights into how medical staff interact with the product, their challenges, and how design modifications can improve these interactions. Clinicians provide invaluable feedback on the ergonomic design, interface usability, and functionality of medical devices, directly impacting the efficacy and safety of these products. This collaborative process ensures that the risk analysis is comprehensive and inclusive, considering a wide range of use cases to propose practical and beneficial solutions to end users.
Business Objectives and Cost/Benefit Analysis
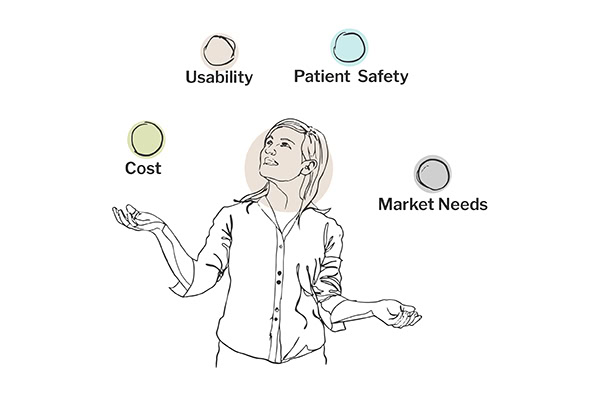
Balancing the imperative of patient safety with economic viability is a critical aspect of product development, particularly in the medical device industry. Our approach to URRA ensures that risk mitigation efforts do not result in prohibitively high costs that could limit the product’s accessibility or feasibility.
For instance, trade-offs are often required to balance advanced functionalities and cost-effectiveness in developing complex machinery like a dialysis machine. These trade-offs may involve selecting materials that meet safety requirements without significantly driving up costs or choosing manufacturing processes that streamline production while maintaining quality standards. Our negotiations and decisions are guided by the principle of achieving the optimal balance between cost, usability, patient safety, and market need. This approach ensures regulatory compliance and addresses the business goals of producing a competitive and financially viable and the market’s clinical goals of getting treatment out to patients.
Iterative Approaches and Market Needs
 In some instances, particularly those involving life-saving treatments, the speed of bringing a product to market can be as critical as the product’s features. Our URRA process supports an iterative development approach in these literal life-and-death scenarios. This strategy prioritizes the essential functions and safety features necessary for the initial release, with plans to enhance certain features that improve user-friendliness.
In some instances, particularly those involving life-saving treatments, the speed of bringing a product to market can be as critical as the product’s features. Our URRA process supports an iterative development approach in these literal life-and-death scenarios. This strategy prioritizes the essential functions and safety features necessary for the initial release, with plans to enhance certain features that improve user-friendliness.
This phased approach to product release allows us to meet urgent market needs without compromising on fundamental safety requirements. It provides patients early access to crucial treatments while allowing manufacturers to refine the product. Such an approach is particularly suited to fast-evolving fields where delaying product launch could have significant human costs.
It’s worth noting that the particulars of our phased approach and HFE activities are not performed the same way every time, and we do not use the same level of rigor for every project. We take a pragmatic view of product development, factoring in the larger business and market context to make improvements throughout the product lifecycle. By planning for iterative updates, we can incorporate user feedback and technological advances to enhance a product’s performance and usability. These updates ensure that the product meets current needs and is adaptable to future demands and changes in the healthcare landscape.
____
OTHER ARTICLES IN THIS SERIES
If you missed any previous articles in this series on appropriately assessing use-related risk and its value to your medical device, service, company, customers, and end users, you can find them here:
4. The Importance of Continuous Risk Assessment in Medical Device Design & Development
____
Ready to Scale and Conduct Use-Related Risk Effectively?
Please submit an inquiry using the below form. A member of our team will contact you shortly.
____
HUMAN FACTORS ENGINEERING AT THRIVE
THRIVE’s Human Factors professionals have decades of experience applying Human Factors to products ranging from medical devices used by specialized healthcare professionals in clinical environments to combination products used by laypeople in the home.
If it’s your first time applying Human Factors and you need a comprehensive end-to-end Human Factors program, we’ll scope out the program and conduct the activities on your behalf, leaving you time and resources to focus elsewhere. If you’re a resource-constrained HFE professional, we’ll provide the teamwork, collaboration, and support to help you meet your goals. Or, if you want a final sanity check to ensure you’ve met the latest and greatest expectations, we’ll do that, too.
ATLANTA | CHICAGO